The volume of the Cl₂ gas is 4.15 L.
Step-by-step explanation:
This problem can be easily solved using the ideal gas law.
As in ideal gas law, it is stated that the product of pressure and volume is equal to the product of number of moles with temperature with gas constant.
PV=nRT
So, in the present case, pressure P is given as 177 kPa, temperature T = 348 K and the mass of chlorine gas is given as 18 g.
So first step, we have to determine the value of 'n' using the mass of Chlorine gas given. The mass should be converted to moles by dividing the mass with molar mass of the gas.
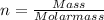
As molar mass of Cl is 35.453 g/mol, then

So,
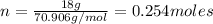
Then, the volume can be determined with R = 8.3144×10³ L Pa K⁻¹ mol⁻¹ as shown below:
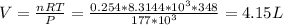
Thus, the volume of the Cl₂ gas is 4.15 L.