The total pressure of gas samples are 102.69 atmosphere, if the gas samples have pressure of 12.3 atmosphere, 56.7 atmosphere, 1.09 atmosphere, and 32.6 atmosphere.
Step-by-step explanation:
The given is,
Gas samples pressure are,
12.3 atmosphere, 56.7 atmosphere, 1.09 atmosphere, and 32.6 atmosphere
Step:1
From the Dalton’s Law of Partial Pressure,
The total pressure of a mixture of gases can be defined as the sum of the pressures of each individual gas,
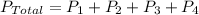 .........................(1)
.........................(1)
Where,
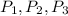 ., etc. are the pressure of gas sample respectively 1,2,3.,etc.
., etc. are the pressure of gas sample respectively 1,2,3.,etc.
Step:2
From the given,
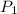 = 12.3 atmosphere
= 12.3 atmosphere
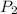 = 56.7 atmosphere
= 56.7 atmosphere
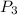 = 1.09 atmosphere
= 1.09 atmosphere
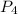 = 32.6 atmosphere
= 32.6 atmosphere
Equation (1) becomes,
= 12.3 + 56.7 + 1.09 + 32.6
= 102.69 atmosphere
Total pressure of gas samples are = 102.69 atmosphere
Result:
The total pressure of gas samples are 102.69 atmosphere, if gas samples have pressure of 12.3 atmosphere, 56.7 atmosphere, 1.09 atmosphere, and 32.6 atmosphere.