The percent yield is 71.3 %.
Step-by-step explanation:
Percent yield is the measure to analyze the success percentage of any experiment .The percent yield of any experiment can be obtained by the ratio of actual or experimental value to expected or theoretical value multiplied with 100.
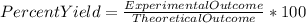
So, in the present problem, we have obtained 10.7 g of adamantium nitrate from Wolverine's 10 pound claws. So the actual value or the experimental value is the amount of adamantium nitrate obtained from Wolverine's claws.
Thus, the experimental outcome is 10.7 g. While we had expected to recover 15 g of adamantium nitrate. So the theoretical outcome is 15 g.
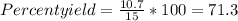
Thus, the percent yield is 71.3 %.