Answer : The correct option is, The equilibrium will shift to the left to favor the reactants.
Explanation :
Reaction quotient (Q) : It is defined as the measurement of the relative amounts of products and reactants present during a reaction at a particular time.
The given balanced chemical reaction is,
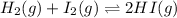
The expression for reaction quotient will be :
![Q=([HI]^2)/([H_2][I_2])](https://img.qammunity.org/2021/formulas/chemistry/middle-school/mphtz5m1jwp4dke4g6ljjn5ezpxjev5fk2.png)
In this expression, only gaseous or aqueous states are includes and pure liquid or solid states are omitted.
The given reaction quotient value is,
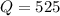
The given equilibrium constant value is,

Equilibrium constant : It is defined as the equilibrium constant. It is defined as the ratio of concentration of products to the concentration of reactants.
There are 3 conditions:
When
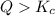 that means product > reactant. So, the reaction is reactant favored.
that means product > reactant. So, the reaction is reactant favored.
When
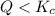 that means reactant > product. So, the reaction is product favored.
that means reactant > product. So, the reaction is product favored.
When
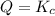 that means product = reactant. So, the reaction is in equilibrium.
that means product = reactant. So, the reaction is in equilibrium.
From the above we conclude that, the
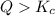 that means product > reactant. So, the reaction is reactant favored that means reaction must shift to the reactant (left) to be in equilibrium.
that means product > reactant. So, the reaction is reactant favored that means reaction must shift to the reactant (left) to be in equilibrium.
Hence, the correct option is, The equilibrium will shift to the left to favor the reactants.