Answer: The total amount of energy needed overall in kilojoules is, 21.8
Step-by-step explanation:
The conversions involved in this process are :
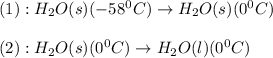
Now we have to calculate the enthalpy change.
![\Delta H=[m* c_(s)* (T_(final)-T_(initial))]+n* \Delta H_(fusion)](https://img.qammunity.org/2021/formulas/chemistry/high-school/b2a3ys9p4ua4134g2vyksb0u6kums07050.png)
where,
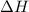 = enthalpy change = ?
= enthalpy change = ?
m = mass of ice = 47.7 g = 0.0477 kg (1kg=1000g)
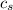 = specific heat of solid water =
= specific heat of solid water =
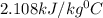
n = number of moles of water =
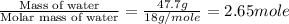
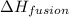 = enthalpy change for fusion = 6.01 kJ/mole
= enthalpy change for fusion = 6.01 kJ/mole
Now put all the given values in the above expression, we get
![\Delta H=[0.0477kg* 2.108kJ/kg^0C* (0-(-58))^0C]+2.65mole* 6.01kJ/mole](https://img.qammunity.org/2021/formulas/chemistry/high-school/brijbv0pzv0d9gr1ps0x49m9zr0n3lx3dz.png)
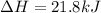
Therefore, the enthalpy change is, 21.8 kJ