The new volume at standard temperature and pressure is 5.08 L.
Step-by-step explanation:
As per the kinetic theory of gases, the volume occupied by gas molecules will be inversely proportional to the pressure of the gas molecules. This is termed as Boyle's law.
So, pressure∝
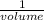
Thus, if two pressure and two volumes are given then,
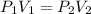
Now, we known the values of P₁ = 8 atm, V₁ = 635 mL, P₂ = 1 atm and V₂ we have to determine. We are considering P₂ = 1 atm, because we have to determine V₂ at standard temperature and pressure. And standard pressure is 1 atm.
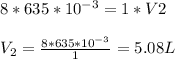
Thus, the new volume at standard temperature and pressure is 5.08 L.