Answer:
It would take 24 minutes for the element to decay to 50 grams
Explanation:
The equation for the amount of the element present, after t minutes, is:
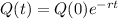
In which Q(X) decays radioactively with a half life of 12 minutes.(0) is the initial amount and r is the rate it decreases.
Half life of 12 minutes
This means that
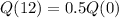
So
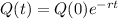
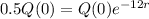
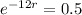
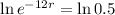
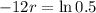

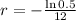
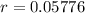
If there are 200 grams of Element X, how long, to the nearest tenth of a minute, would it take the element to decay to 50 grams?
This is t when Q(t) = 50. Q(0) = 200.
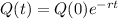
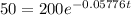
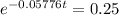
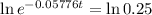
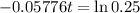
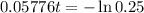
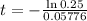
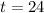
It would take 24 minutes for the element to decay to 50 grams