Answer: No, it cannot
Step-by-step explanation:
Rate law says that rate of a reaction is directly proportional to the concentration of the reactants each raised to a stoichiometric coefficient determined experimentally called as order.
Elementary reactions are reactions which takes place in single step. For elementary reactions the order is same as the stoichiometric coefficients.
Complex reactions are reactions that take place in more than one step and rate is determined by the slowest step of the reaction.
The given reaction is:
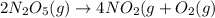
Rate law is:
![Rate=k[N_2O_5]^1](https://img.qammunity.org/2021/formulas/chemistry/high-school/6bsay9d6p0x456ok4v008wye558fl75b5i.png)
As order is not same as the stoichiometric coefficient of
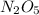 , it is a complex reaction.
, it is a complex reaction.