Answer:
The correct answer is option 3.
Step-by-step explanation:
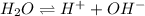
The expression of
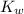 is given by :
is given by :
![K_w=[H^+][OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/7jdjcuyzjoh0xkeaez8b0dl2066xe1tebt.png)
![K_w\propto [H^+]](https://img.qammunity.org/2021/formulas/chemistry/high-school/i4nrg317hfpom6fuam5ukl9pbz45xe57lu.png)
![K_w\propto [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/jizrd11izmd1qn6v5e0dho7fkrnw3ad25a.png)
Rise in temperature will result in more dissociation of water molecules into hydrogen ions and hydroxide ions.
With an increase in concentration of hydrogen ions and hydroxide ions value of
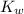 will also increase.
will also increase.