15.3 litres of water will be produced if we take 1.7 litres of Hydrogen
Step-by-step explanation:
Let's take a look over synthesis reaction;
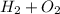

Balancing the chemical reaction;
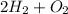
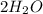
Thus, 2 moles of hydrogen molecules are required to form 2 moles of water molecules.
Equating the molarity;
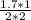 =
=
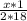
(Since, the molecular mass of hyd and water is 2 and 18 respectively)
x=
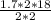
x= 15.3 litres.
Thus,15.3 L of water will be produced if we take 1.7 litres of Hydrogen in a synthesis reaction.