Oxygen is the limiting reagent in the following synthesis reaction.
Step-by-step explanation:
At first, we have to balance the chemical reaction,
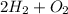
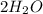
i.e.,oxygen molecule requires 2 moles of hydrogen molecule in order to synthesise 2 moles of water molecule.
no. of moles of
 =
=
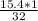
=0.48125 moles
no. of moles of
 =
=
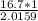
= 8.2841 moles.
Now, for 1 mole of oxygen,2 mole of hydrogen is used, so;
no. of moles of
 = 8.2841/2 = 4.142 moles.
= 8.2841/2 = 4.142 moles.
But, we don't have that many moles of
 and that's why, oxygen is considered as limiting reagent.
and that's why, oxygen is considered as limiting reagent.