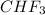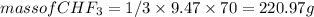Answer:
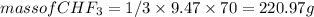
Step-by-step explanation:
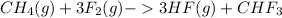
As given in the question that methane(
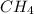 ) is taken excess amount
) is taken excess amount
mass of
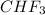 depend only on mass of fluorine
depend only on mass of fluorine
mass of
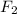 =180 g
=180 g
mole of
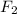 =
=
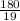 =9.47
=9.47
3 mole of
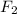 gives 1 mole of
gives 1 mole of
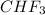
so 1 mole will give
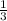 mole of
mole of
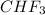
therefore,
9.47 mole of
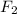 will give
will give
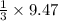 mole of
mole of