Answer: a) pH of a 0.1 M vinegar solution is 2.9
b) It is an acid as pH is less than 7
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pH is calculated by taking negative logarithm of hydrogen ion concentration.
Acids have pH ranging from 1 to 6.9, bases have pH ranging from 7.1 to 14 and neutral solutions have pH equal to 7.
As vinegar is a weak acid, its dissociation is represented as;
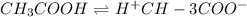
cM 0 0

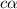
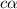
So dissociation constant will be:
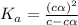
Give c= 0.1 M
![[H^+]=c* \alpha](https://img.qammunity.org/2021/formulas/chemistry/college/6iel1ors69hl9ilvy1vx41nw46k5uyhjc0.png)
![[H^+]=0.1* \alpha](https://img.qammunity.org/2021/formulas/chemistry/high-school/odvgiigdxlmsgdtsk5rl1kq19z1s4oz1pn.png)
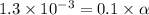
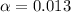
Also
![pH=-log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/wyj0nahkywle04sx44478osqilvygxax2t.png)
![pH=-log[1.3* 10^(-3)]=2.9](https://img.qammunity.org/2021/formulas/chemistry/high-school/74tvaepdolt5piko4yfaf4spdi0s5biihq.png)
Thus pH of a 0.1 M vinegar solution is 2.9
As pH is less than 7, it is an acid.