Answer:
The correct answer is option a.
Step-by-step explanation:
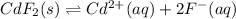
Equilibrium concentration cadmium ions =
![[Cd^(2+)]=0.0585 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/w76v0x7hecxvvi1zi4qo1wyn0t3lvq7saz.png)
Equilibrium concentration fluoride ions =
![[F^(-)]=0.117 M](https://img.qammunity.org/2021/formulas/chemistry/high-school/kqot98e0p4kjueam58ikeb02mvatq0xcle.png)
Molar solubility is the maximum concentration of salt present in water in ionic form beyond that no more salt will exist in its ionic form and will settle down in bottom of the solution.
The molar solubility of the solid cadmium fluoride = 0.0585 M
 ..[1]
..[1]
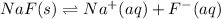
Due to addition of sodium fluoride will increase concentration of fluoride in the solution.And due to common ion effect the equilibrium will shift in backward direction in [1], that is precipitation of more cadmium fluoride.
Hence, decrease in solubility will be observed.