Answer:
1.5V
Step-by-step explanation:
We can solve this problem by using the equation of state for an ideal gas, which is:
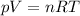
where
p is the pressure of the gas
V is its volume
n is the number of moles
R is the gas constant
T is the absolute temperature of the gas
In this problem, the pressure and the temperature of the gas are held constant: so we can rewrite the equation as
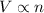
And so:
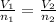
Where here we have:
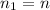 is the initial number of moles
is the initial number of moles
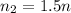 is the final number of moles
is the final number of moles
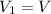 is the initial volume of the gas
is the initial volume of the gas
Solving for V2, we find the new volume:
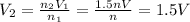
So, the volume of the gas increases by 1.5 times.