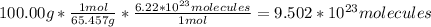Answer:
9.5 * 10^23 molecules of HCl
Explanation:
just a note this might be better to put in the chem section next time since this is a stoichiometry question
the molar mass of HCL is 1.007 + 35.45 = 65.457 g/mol
to find the number of moles, divide 100 by 65.457
then multiply the number of moles by 6.22 * 10^23 to get the number of molecules