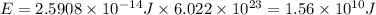Answer:
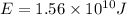
Step-by-step explanation:
The energy of a photon, E, can be calculated with the Planck-Einstein equation:
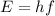
Where:
- h is Planck's constant 6.626×10⁻³⁴ J.s, and
- f is the frequency of the photon or electromagnetic radiation.
Substituting with your data:
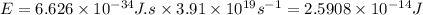
Now multiply by Avogadro's number to obtain the energy of one mole of photons: