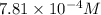Answer: The concentration of
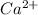 ions in the solution is
ions in the solution is
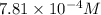
Step-by-step explanation:
We are given:
Concentration of hydroxide ion =
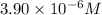
Solubility product is defined as the product of concentration of ions present in a solution each raised to the power its stoichiometric ratio.
The equation for the ionization of the hydroxyapatite is given as:
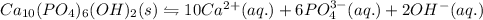
10s 6s
The expression for the solubility product of hydroxyapatite will be:
![K_(sp)=[Ca^(2+)]^10[PO_4^(3-)]^6[OH^-]^2\\\\K_(sp)=(10s)^(10)* (6s)^6* (3.90* 10^(-6)+2s)^2=4.6656* 10^(14)* s^(16)* (3.90* 10^(-6)+2s)^2](https://img.qammunity.org/2021/formulas/chemistry/college/l64kkt407q3fq2wyina6evtiazph4p8s25.png)
We are given:
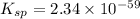
Putting values in above equation, we get:
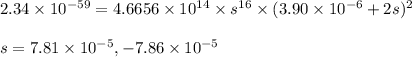
Neglecting the negative value of 'x' because concentration cannot be negative.
So, the concentration of calcium ions in the solution = 10s =
![[10* (7.81* 10^(-5))]=7.81* 10^(-4)M](https://img.qammunity.org/2021/formulas/chemistry/college/8zriyre20cy8himbj6y6v3it6mlu238mpz.png)
Hence, the concentration of
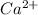 ions in the solution is
ions in the solution is