Answer:
A
Step-by-step explanation:
Recall that a mole of any substance contains 6.02 × 10²³ amounts of that substance.
In other words, a mole of gold atoms contains 6.02 × 10²³ gold atoms.
Hence, by dimensional analysis, we have that:
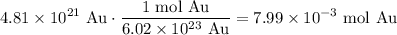
Therefore, there is about 7.99 × 10⁻³ moles of gold present in the sample.
In conclusion, the answer is A.