Answer: The molar solubility of
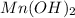 in a solution that is buffered at ph 8.00 is 0.19 M
in a solution that is buffered at ph 8.00 is 0.19 M
Step-by-step explanation:
Solubility product is defined as the equilibrium constant in which a solid ionic compound is dissolved to produce its ions in solution. It is represented as

We are given:
Solubility product of
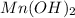 =
=
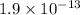
The equation for the ionization of the
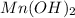 is given as:
is given as:
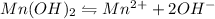
1 mole of
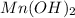 gives 1 mole of
gives 1 mole of
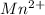 and 2 moles of
and 2 moles of

Given : pH = 8.00
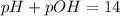
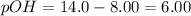
![6.00=-log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/13ry2h259iqxexy6bcz6y0keaikl7xh0y8.png)
![[OH^-]=10^(-6)M](https://img.qammunity.org/2021/formulas/chemistry/high-school/pd77452qcdcwfeqopf7wuqcuivjteahm5w.png)
![K_(sp)=[Mn^(2+)][OH^(-)]^2](https://img.qammunity.org/2021/formulas/chemistry/high-school/zn4td4ebtl29nbizvs0waqgm0de22nahx5.png)
![1.9* 10^(-13)M=[s][(10^(-6))^2]](https://img.qammunity.org/2021/formulas/chemistry/high-school/7fyjg7pxwugsnnucbrl4owf9q6jfz9mir4.png)
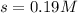
Thus the molar solubility of
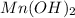 in a solution that is buffered at ph 8.00 is 0.19 M
in a solution that is buffered at ph 8.00 is 0.19 M