Answer: -19 kJ
Step-by-step explanation:
The change in enthalpy
 is mathematically expressed as the difference between the the total potential energy of products
is mathematically expressed as the difference between the the total potential energy of products
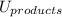 and the potential energy of the reactants
and the potential energy of the reactants
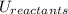 :
:
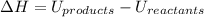
Where:
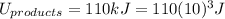
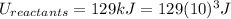
Then:
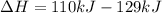
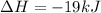 The negative sign in this result means we have a negative enthalpy, hence an exothermic reaction (where heat is released).
The negative sign in this result means we have a negative enthalpy, hence an exothermic reaction (where heat is released).