Answer:
604.13L
Step-by-step explanation:
Given parameters:
Volume of propane = 200dm³ or 200L
Unknown:
Volume of carbon dioxide formed = ?
Solution:
The reaction equation is given as;
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
The above is the balanced reaction equation.
Let us solve from the known to the unknown;
The known here is propane and unknown is carbon dioxide
At STP;
Number of moles of propane =
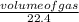 =
=
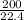 = 8.93moles
= 8.93moles
From the balanced reaction equation;
1 mole of propane gives 3 moles of carbon dioxide;
8.93 moles of propane will produce; 3 x 8.93 = 26.97moles
Now,
Volume of carbon dioxide = number of moles x 22.4
= 26.97 x 22.4
= 604.13L