Answer:
12 moles of MgO
1.8 moles of O₂
5.5 moles of Mg
Step-by-step explanation:
These questions are all related and they involve comparing the number of moles of reactant to products.
Here we use molar relationship because they express what happens in a reaction much more better.
Problem 1:
2Mg + O₂ → 2MgO
We work from the known to the unknown;
The known is oxygen gas; which is 6moles
Unknown is MgO;
1 mole of O₂ produced 2 mole of MgO
6 moles of O₂ will produce (6 x 2) moles of MgO
= 12 moles of MgO
Problem 2:
2Mg + O₂ → 2MgO
Known; 3.6 moles of Magnesium
Unknown; number of moles of oxygen;
2 moles of Mg reacted with 1 mole of O₂
3.6 moles of Mg will react with
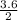 = 1.8 moles of O₂
= 1.8 moles of O₂
Problem 3:
2Mg + O₂ → 2MgO
Known: number of of moles of MgO = 5.5moles
Unknown: number of moles of Mg = ?
2 moles of MgO will be produced from 2 moles of Mg
5.5 moles of MgO will be produced from
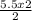 = 5.5 moles of Mg
= 5.5 moles of Mg