Answer:
0.05M or 0.05moldm⁻³
Step-by-step explanation:
Mass of NaHSO₄ = 12g
Volume of water = 2L
Unknown:
Molarity of the solution = ?
Solution:
The molarity of a solution is the amount of moles of solute dissolved in a given volume of solvent.
Mathematically;
Molarity =
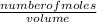
Let us find the number of moles of NaHSO₄;
Number of moles =
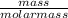
molar mass of NaHSO₄ = 23 + 1 + 32 + 4(16) = 120g/mol
Number of moles of NaHSO₄ =
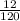 = 0.1mole
= 0.1mole
Now;
Molarity =
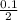 = 0.05M or 0.05moldm⁻³
= 0.05M or 0.05moldm⁻³