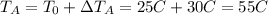Answer:
55 C
Step-by-step explanation:
When a certain substance is heated, the temperature of the substance increases according to the equation
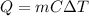
where
Q is the heat supplied to the substance
m is the mass of the substance
C is the specific heat of the substance
 is the change in temperature of the substance
is the change in temperature of the substance
The equation can be rewritten as
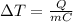
In this problem, the two pots both contain water, so the specific heat (C) is the same. Also, the same amount of heat is supplied, so Q is the same.
However, pot A contains 1 liter of water, while pot B containes 3 liters: this means that the mass of water in pot B is 3 times the mass of water in pot A,
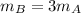
Since
 is inversely proportional to the mass, this means that
is inversely proportional to the mass, this means that
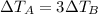
The change in temperature of the water in pot B is
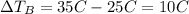
So, the change in temperature of the water in pot A is
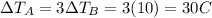
So, the final temperature of pot A is