Answer : The
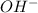 concentration is, 0.0316 M
concentration is, 0.0316 M
Explanation :
pH : It is defined as the negative logarithm of hydrogen ion concentration.
First we have to calculate the pOH.
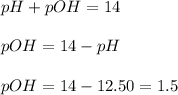
Now we have to calculate the
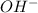 concentration.
concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hdm1ob4dj6mx2sy3kobrrj91lzbh3927bk.png)
![1.5=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/middle-school/aq601r7bftdyw1614zwhx6q07kypbjw13b.png)
![[OH^-]=0.0316M](https://img.qammunity.org/2021/formulas/chemistry/middle-school/x3vti0xbqt5m2ufmoxn5xquhmhut02e6ph.png)
Therefore, the
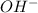 concentration is, 0.0316 M
concentration is, 0.0316 M