Answer: The balanced chemical equation for the combustion of liquid ethanol is written below.
Step-by-step explanation:
Combustion reactions are defined as the reactions in which a hydrocarbon reacts with oxygen gas to produce carbon dioxide and water. Heat is released during these reactions.
A balanced chemical equation is defined as the equation in which total number of individual atoms on the reactant side is equal to the total number of individual atoms on product side.
The balanced chemical equation for the combustion of liquid ethanol follows:
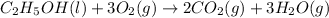
By Stoichiometry of the reaction:
1 mole of liquid ethanol reacts with 3 moles of oxygen gas to produce 2 moles of carbon dioxide gas and 3 moles of water vapor.
Hence, the balanced chemical equation for the combustion of liquid ethanol is written above.