Answer:
0.02kg
Step-by-step explanation:
Given parameters:
Temperature of ice = 0°C
Energy = 6.8 x 10³J(let us assume it is 10³)
Unknown:
Mass of ice melted = ?
Solution:
This problem deals with a change of state physical change. The ice changes to water at the same temperature.
Heat changes accompanying such reactions is given by;
H = mL
where m is the mass
L is the heat of vaporization
For water L = 3.3×10⁵ joule/kg
Since m is the unknown; we can solve for it;
m =
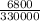 = 0.02kg
= 0.02kg