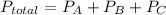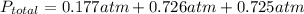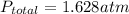Answer:
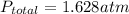
Step-by-step explanation:
According to dalton theory of partial pressure , partial pressure applied by a component in a mixture of more gas is same as pressure applied when it is taken alone in the container.
And thus
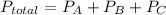
and P_A and P_B is given so we need to find P_C for the 3rd gas,
and we will solve by assuming that gas follows the ideality nature of gas,
PV=nRT
P_A=0.177atm
P_B=0.726atm
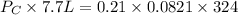
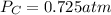
therefore,