Answer: The molarity of solution will be 0.00325 M
Step-by-step explanation:
Molarity : It is defined as the number of moles of solute present per liter of the solution.
Formula used :
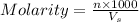
where,
n= moles of solute =
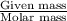
given mass = 239 mg = 0.239 g (1g=1000mg)
moles of
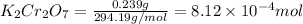
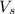 = volume of solution in ml = 250 ml
= volume of solution in ml = 250 ml
Now put all the given values in the formula of molarity, we get
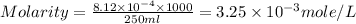
Therefore, the molarity of solution will be 0.00325 M