Answer:
32.4%
Step-by-step explanation:
Given parameters:
Mass of hydrated sample = 25g
Mass of anhydrous salt = 16.9g
Unknown:
Percentage of water in the hydrate = ?
Solution:
Obviously, there is a mass difference between the original hydrated sample and anhydrous salt which should indicate clearly that the mass loss is due to the evaporated water from the salt.
Mass of salt of water = mass of hydrated salt - mass of anhydrous salt
= 25g - 16.9g
= 8.1g
Percentage of water =
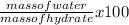
=
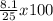
= 32.4%