Answer:
a)
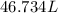 of hard water is require to produce
of hard water is require to produce
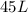 of pure water.
of pure water.
b) Same water would not work for purifying sea water as it would require so much of energy.
Step-by-step explanation:
a)
Osmotic pressure of a solution is calculated by the formula,
Π=
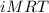
Where,
Π is the osmotic pressure
 is van't hoff factor
is van't hoff factor
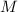 is molar concentration (mol/L)
is molar concentration (mol/L)
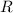 is gas constant
is gas constant
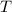 is Temperature in Kelvin
is Temperature in Kelvin
Now,
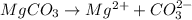
So, according to the above equation
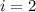
Calculation concentration at which reverse osmosis will stop
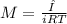
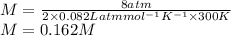
Calculating Initial concentration that is moles of
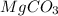
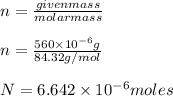
Calculating molarity
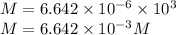
Total number of moles in total volume must remain the same,
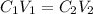
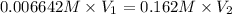
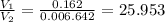
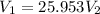
Also,
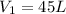
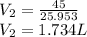
So, net Volume,
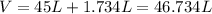
So,
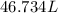 is require to produce
is require to produce
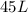 of pure water.
of pure water.
b)
Using the equation for
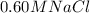 as well,
as well,
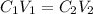
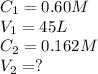
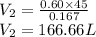
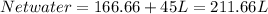
So, the same water would not work for purifying sea water as it would require so much of energy, as we require 211.66L of water to produce 45L of water.