Answer:
The maximum pressure that will be attained in the tank before the plug melts and releases gas is = 36.18 atm
Step-by-step explanation:
Given data
Initial pressure
 = 31.7 atm
= 31.7 atm
Initial Temperature
 = 63.4 °c = 336.4 K
= 63.4 °c = 336.4 K
Final temperature
 = 111 °c = 384 K
= 111 °c = 384 K
From ideal gas equation
P V = m R T
Here Volume , mass & temperature is constant
So pressure is directly proportional to the temperature.
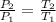
Put all the values in above formula we get
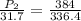
 = 36.18 atm
= 36.18 atm
Therefore the maximum pressure that will be attained in the tank before the plug melts and releases gas.