Answer:
Molar mass of protein is
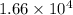 g/mol
g/mol
Step-by-step explanation:
Protein is a non-electrolyte. For a non-electrolyte-
We know,
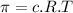
where
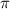 represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.
represents osmotic pressure of solution, c represents molarity of solution and T represents temperature in kelvin scale.
Let's assume molar mass of protein is M g/mol
Then
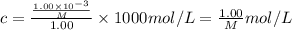
R = 0.0821 L.atm/(mol.K)
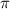 = 1.12 torr = 0.00147 atm
= 1.12 torr = 0.00147 atm
T = (273+25) K = 298 K
So,
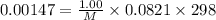
or, M =
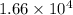
So, molar mass of protein is
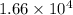 g/mol
g/mol