The equilibrium constant is 0.0022.
Step-by-step explanation:
The values given in the problem is
ΔG° = 1.22 ×10⁵ J/mol
T = 2400 K.
R = 8.314 J mol⁻¹ K⁻¹
The Gibbs free energy should be minimum for a spontaneous reaction and equilibrium state of any reaction is spontaneous reaction. So on simplification, the thermodynamic properties of the equilibrium constant can be obtained as related to Gibbs free energy change at constant temperature.
The relation between Gibbs free energy change with equilibrium constant is ΔG° = -RT ln K
So, here K is the equilibrium constant. Now, substitute all the given values in the corresponding parameters of the above equation.
We get,
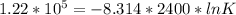
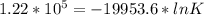
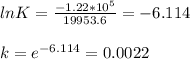
So, the equilibrium constant is 0.0022.