Answer:
The final volume of the gas is 1.29 L
Step-by-step explanation:
To calculate the amount of work done for an isothermal process is given by the equation:
w = work done by the system =

P = pressure = 783 torr = 1.03 atm (1atm=760 torr)
w= 126.6 J = 1.2517Latm (1Latm=101.3J)
 = initial volume = 67.4 ml = 0.0674L (1L=1000ml)
= initial volume = 67.4 ml = 0.0674L (1L=1000ml)
 = final volume = ?
= final volume = ?
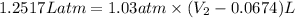
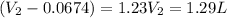
Therefore, the final volume of the gas is 1.29 L