Answer:
1.7L
Step-by-step explanation:
Given parameters:
Volume of hydrogen gas = 1.7L
Unknown:
Volume of water produced = ?
Solution:
A synthesis reaction is a combination reaction that involves two or more substances combining together to give a product.
In the synthesis of water, hydrogen gas and oxygen gas are reacted to give water as a product according to the expression below;
2H₂ + O₂ → 2H₂O
Assuming that the reaction takes place at STP, it is easier to solve this problem.
Now, oxygen gas must be the excess reactant because it is freely available.
Find the number of moles of the known specie first,
Number of moles =
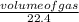
Number of moles of H₂ =
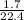 = 0.076moles
= 0.076moles
From the balanced reaction equation;
2 moles of H₂ produced 2 moles of H₂O
0.076 moles of H₂ will produce 0.076 moles of H₂O;
Volume of water produced = number of moles x 22.4
= 0.076 x 22.4
= 1.7L