The given question is incomplete. The complete question is :
Gaseous butane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . If 1.31g of water is produced from the reaction of 4.65g of butane and 10.8g of oxygen gas, calculate the percent yield of water. Be sure your answer has the correct number of significant digits in it.
Answer: 28.0 %
Explanation:
To calculate the moles :
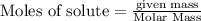
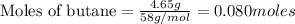
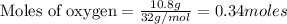
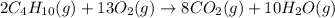
According to stoichiometry :
13 moles of
 require 2 moles of butane
require 2 moles of butane
Thus 0.34 moles of
 will require=
will require=
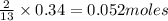 of butane
of butane
Thus
 is the limiting reagent as it limits the formation of product and butane is the excess reagent.
is the limiting reagent as it limits the formation of product and butane is the excess reagent.
As 13 moles of
 give = 10 moles of
give = 10 moles of

Thus 0.34 moles of
 give =
give =
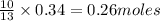 of
of

Mass of
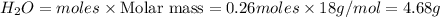
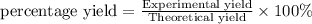
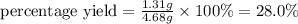
The percent yield of water is 28.0 %