Answer:
Numbe of mole of MgO form=12
Step-by-step explanation:
First calculate the number of mole of
 produced from 4 mole of
produced from 4 mole of
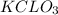
Balance the first reaction:
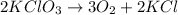
from the above balanced reaction it is clearly that,
by unitry method,
2 mole of
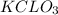 produced 3 mole of
produced 3 mole of

1 mole of
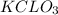 produced 1.5 mole of
produced 1.5 mole of

from 4 mole of
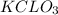
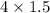 mole of
mole of
 produced
produced
hence we have 6 mole of
 and this total oxygen will react with Mg
and this total oxygen will react with Mg
Balance the second reaction:
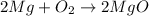
since produced oxygen in first reaction is reacted completely with Mg means Mg is given in excess quantity,
From the second balanced reacion it is clearly that,
1 mole of
 produced 2 Mole of MgO
produced 2 Mole of MgO
hence 6 mole of
 will produce 12 mole of MgO
will produce 12 mole of MgO
Numbe of mole of MgO form=12