Answer:
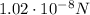 , repulsive
, repulsive
Step-by-step explanation:
The magnitude of the electric force between two charged particles is given by Coulomb's law:
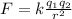
where:
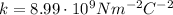 is the Coulomb's constant
is the Coulomb's constant
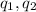 are the two charges of the two particles
are the two charges of the two particles
r is the separation between the two charges
The force is:
- repulsive if the two charges have same sign
- Attractive if the two charges have opposite signs
In this problem, we have two electrons, so:
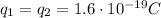 is the magnitude of the two electrons
is the magnitude of the two electrons
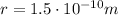 is their separation
is their separation
Substituting into the formula, we find the electric force between them:
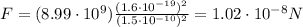
And the force is repulsive, since the two electrons have same sign charge.