Answer : The value of
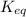 of this reaction is,
of this reaction is,
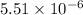
At equilibrium, [L-malate] > [oxaloacetate]
Explanation :
The relation between the equilibrium constant and standard Gibbs free energy is:
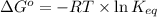
where,
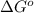 = standard Gibbs free energy = +30 kJ/mol = +30000 J/mol
= standard Gibbs free energy = +30 kJ/mol = +30000 J/mol
R = gas constant = 8.314 J/K.mol
T = temperature =
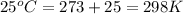
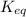 = equilibrium constant = ?
= equilibrium constant = ?
The given reaction is:
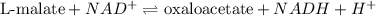
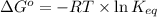
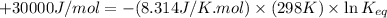
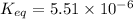
Therefore, the value of
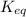 of this reaction is,
of this reaction is,
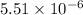
As, the value of
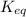 < 1 that means the reaction mixture contains reactants.
< 1 that means the reaction mixture contains reactants.
At equilibrium, [L-malate] > [oxaloacetate]