Answer:
250 mL
Step-by-step explanation:
These are the variable one has to have in mind:
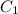 = 16 M
= 16 M
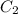 = 2 M
= 2 M
 = 2 L = 2000 mL
= 2 L = 2000 mL
Note: C = concentration; V = volume; subscript 1 = stock solution; subscript = 2 = dilution.
What we have to solve is for

Use the following equation:
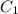 x
x
 =
=
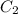 x
x
 ⇒
⇒
 =
=
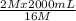 ⇒
⇒
 = 250 mL
= 250 mL