Answer:
The reaction is not in equilibrium and it proceed in reverse direction to reach the equilibruim condition
Step-by-step explanation:
We have
H₂(g) + x₂(g) ⇆ 2HX (g)
Kc = 2.4 x 10 ⁻³ at 25°C
![K_c = ([HX]^2)/([H_2][X_2])](https://img.qammunity.org/2021/formulas/chemistry/college/axay9pwdvf25uwwiiw58rjpivqai26nou9.png)
Now we have 3L reactor and the number of mole of species are 0.150 mole H₂, 0.150 mole X₂ and 0.600 mole HX
Therefore,
![[H_2] = (0.150)/(3) \\= 0.05M](https://img.qammunity.org/2021/formulas/chemistry/college/d97tskeos6mglez1rcdfdl52n38rldidtg.png)
![[X_2] = (0.150)/(3) \\= 0.05M](https://img.qammunity.org/2021/formulas/chemistry/college/v45h5jgpx23qv57vj1jyh6gzxwbss6s912.png)
![[HX] = (0.600)/(3) \\= 0.2M](https://img.qammunity.org/2021/formulas/chemistry/college/3i5qt59xq58t3nrcabbaqo08vire2dhr46.png)
Therefore,
![Q_c = ([HX]^2)/([H_2][X_2])](https://img.qammunity.org/2021/formulas/chemistry/college/jhmgomrrmjlwx5wd44ed2f1q3qnwkysuoc.png)
![Q_c = ([0.200]^2)/([0.05][0.05])\\\\=16](https://img.qammunity.org/2021/formulas/chemistry/college/kjdn1aanc49ntvaif2zi76yp09v314s0az.png)
Now, we calculate the free energy change for the reaction
ΔrG = RT In Q/K
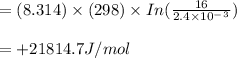
As free energy is +ve so the reaction is not in equilibruim and proceed in reverse reaction to reach tje equilibruim condition