Answer:
Percentage purity of
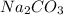 in sample is 17.13%
in sample is 17.13%
Step-by-step explanation:
Net Ionic equation:
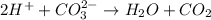
So, two moles of
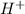 neutralize 1 mol of
neutralize 1 mol of
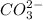
Alternatively, two moles of HCl neutralize 1 mol of
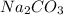
Number of moles of HCl added =
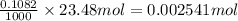
So, 0.002541 moles of HCl neutralizes 0.0012705 moles of
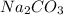
Molar mass of
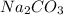 = 105.99 g/mol
= 105.99 g/mol
So, mass of
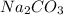 present in impure sample =
present in impure sample =
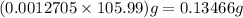
So percentage purity of
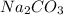 in sample =
in sample =
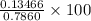 % = 17.13%
% = 17.13%