Answer: produce hydroxide ions
Step-by-step explanation:
Arrhenius acids are substances which dissociate in water to give hydrogen ions.
For example:
 is an Arrhenius acid.
is an Arrhenius acid.
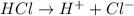
Arrhenius bases are substances which dissociate in water to give hydroxide ions.
For example:
 is an Arrhenius base.
is an Arrhenius base.
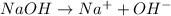
An Arrhenius base is best defined as produce hydroxide ions