Answer:
Initially; 5.22 moles of glucose was initially added to the reactor
The heat produced and subsequently removed from the system = 6.44056 × 10 ¹¹ kJ
Step-by-step explanation:
Given that the unbalanced equation of the reaction is:
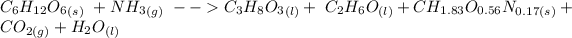
Then the balanced equation can be written as :
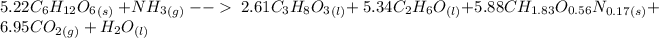
Thus; Initially; 5.22 moles of glucose was initially added to the reactor.
However ; the mass of ethanol = 1.4 lbm
= 635 gram (since 1 lbm = 454 gram)
From stiochiometry ;
number of moles =
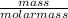
molar mass of ethanol = 46 g/mol
so; the total numbers of moles =
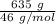
the total numbers of moles = 13.80 moles
However ; for 5.34 moles of ethanol; 5.88 moles of S. cerevisiae is formed:
Now; for 1 mole of ethanol ; we have:
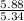 moles of S. cerevisiae formed.
moles of S. cerevisiae formed.
So, for 13.80 moles of ethanol, we wil have
=
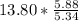 moles of S. cerevisiae formed
moles of S. cerevisiae formed
= 15.19 moles of S. cerevisiae formed.
We are told that the heat of combustion of S. cerevisiae = -21.2 kJ/g
Then by the combustion of S. cerevisiae the heat produced and subsequently removed from the system = 15.19 mole × 21.2 kJ/kg ( molecular weight of S. cerevisiae )
If the molecular weight of S. cerevisiae for the largest DNA strand = 2×10⁹; Then, we have:
= 15.19 mole × 21.2 kJ/kg ( 2 × 10⁹ )
= 6.44056 × 10 ¹¹ kJ