Answer:
- You need to convert the number of atoms of Ca into mass in grams, using Avogadro's number and the atomic mass of Ca.
Step-by-step explanation:
The amount of matter is measured in grams. Thus, you need to convert the number of atoms of Ca (calcium) into mass to compare with 2.45 grams of Mg.
To convert the atoms of calcium into mass, you divide by Avogadro's number, to obtain the number of moles of atoms, and then divide by the atomic mass of calcium.
1. Number of moles, n
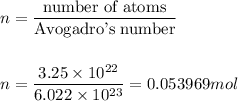
2. Mass
- mass = number of moles × atomic mass
- mass = 0.053969mol × 40.078g/mol = 2.16g
Then, 2.45 g of Mg represent a greaer mass than the 3.25 × 10²² atoms of Ca.