The molarity of Barium Hydroxide is 0.289 M.
Step-by-step explanation:
We have to write the balanced equation as,
Ba(OH)₂ + 2 HNO₃ → Ba(NO₃)₂ + 2 H₂O
We need 2 moles of nitric acid to react with a mole of Barium hydroxide, so we can write the law of volumetric analysis as,
V1M1 = 2 V2M2
Here V1 and M1 are the volume and molarity of nitric acid
V2 and M2 are the volume and molarity of Barium hydroxide.
So the molarity of Ba(OH)₂, can be found as,
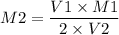
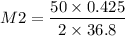
= 0.289 M