Answer:
Time take to deposit Ni is 259.02 sec.
Step-by-step explanation:
Given:
Current
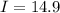 A
A
Faraday constant
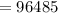
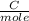
Molar mass of Ni
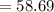
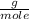
Mass of Ni
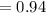 g
g
First find the no. moles in Ni solution,
Moles of Ni
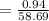
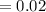 mol
mol
From the below reaction,
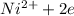 ⇆
⇆
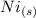
Above reaction shows "1 mol of
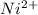 requires 2 mol of electron to form 1 mol of
requires 2 mol of electron to form 1 mol of
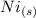 "
"
So for finding charge flow in this reaction we write,

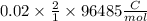
Charge flow
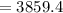 C
C
For finding time of reaction,
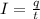
Where
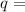 charge flow
charge flow
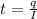
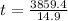
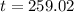 sec
sec
Therefore, time take to deposit Ni is 259.02 sec.