Answer:
Tungsten is used for this experiment
Step-by-step explanation:
This is a Thermal - equilibrium situation. we can use the equation.
Loss of Heat of the Metal = Gain of Heat by the Water
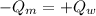
Q = mΔT
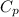
Q = heat
m = mass
ΔT = T₂ - T₁
T₂ = final temperature
T₁ = Initial temperature
Cp = Specific heat capacity
Metal
m = 83.8 g
T₂ = 50⁰C
T₁ = 600⁰C
Cp =

Water
m = 75 g
T₂ = 50⁰C
T₁ = 30⁰C
Cp = 4.184 j.g⁻¹.⁰c⁻¹
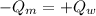
⇒ - 83.8 x
 x (50 - 600) = 75 x 4.184 x (50 - 30)
x (50 - 600) = 75 x 4.184 x (50 - 30)
⇒
 =
=
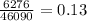 j.g⁻¹.⁰c⁻¹
j.g⁻¹.⁰c⁻¹
We know specific heat capacity of Tungsten = 0.134 j.g⁻¹.⁰c⁻¹
So metal Tungsten used in this experiment